Reactor Types in Aspen HYSYS
In the world of chemical
engineering, process simulation is an important step in designing, analyzing,
and optimizing various chemical processes. Aspen HYSYS is a very powerful and
widely used industrial process simulation software worldwide. One important
aspect of process simulation is the modeling of a chemical reactor that matches
the type of reaction being observed. Below, we will discuss some of the reactor
types that you can encounter within Aspen HYSYS:
1. Conversion Reactor
Conversion reactor simulation in
HYSYS is done by setting the conversion value of a reaction. This type of
reactor is easier to achieve convergence compared to CSTR and PFR reactors.
Users can use it to calculate how much reactants can be converted into products.
Conversion reactors can also be used for a series of continuous reactions,
where the products in the previous reaction can become reactants in the next
reaction. We will make an example of a case that uses a conversion reactor.
Hydrogen production from
hydrocarbons has increased significantly in the last decade. The conversion of
fuel (methane gas) to hydrogen can take place by partial oxidation. This method
involves burning methane with oxygen to produce carbon dioxide and hydrogen, as
shown in the following reaction:
It is known that the conversion
of reaction 1 is 40% (CH4 basis) and reaction 2 is 60% (CH4 basis). Make a
simulation of the above reaction with the specifications of the reactants
entering the reactor as follows.
|
Spesifikasi |
Metana |
Oksigen |
|
Suhu (oC) |
25 |
25 |
|
Tekanan (bar) |
2 |
2 |
|
Laju Alir (Kmol/hr) |
100 |
100 |
|
Mol Fraksi |
||
|
Metana |
1 |
0 |
|
Oksigen |
0 |
1 |
Open the HYSYS Apsen Program and
select all components involved in the process as in the reaction. Use Peng-Robingson
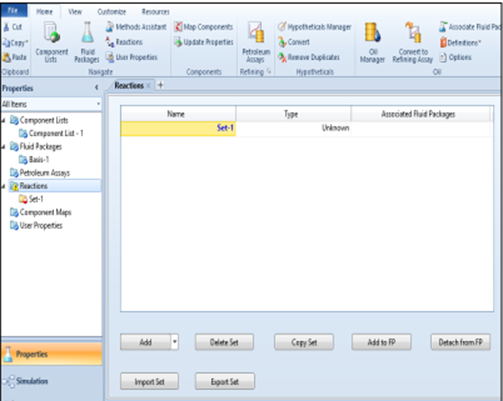
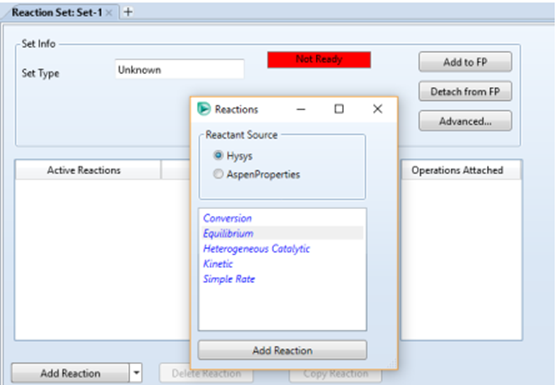
Fluid Package. Click on the "Reaction" option
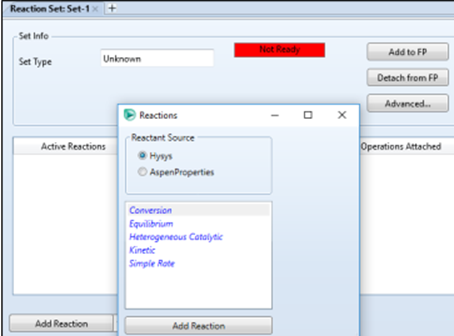
click Add, then Add
Reaction, and select Conversion in the reactions window.
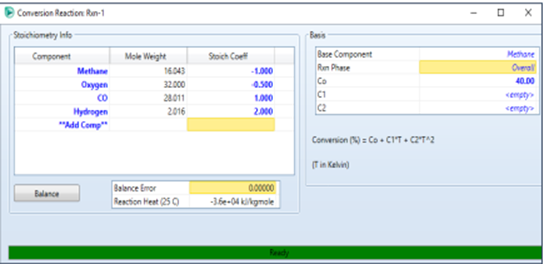
Double-click on RXN-1,
then we fill in the components, stoichiometry, and conversion of
the reaction we want to achieve.
After both reactions are added,
click on the Add to FP → Add Set to Fluid Package option, and the
notification changes from yellow to green (Ready), then enter the simulation
page.
Create a stream according to the specifications
above, then connect it to the conversion reactor (which can be found in the
column section).
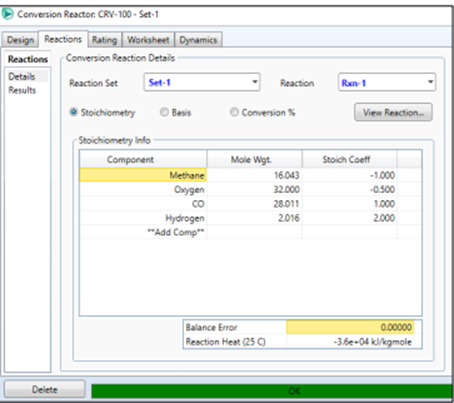
Double click on the reactor icon
→ Reaction, In Reaction Set select Set-1.
To see the reaction output
product, click Worksheet→ Condition or Composition.
2. Equilibrium Reactor
An equilibrium reactor is a
reactor for modeling equilibrium reactions. The outflow from this reactor is in
a chemically and physically balanced state. The number of reaction sets entered
in this reactor operating model can be unlimited, which will be completed by
HYSYS simultaneously or sequentially. In this reactor model, each component or
mixing process does not have to occur ideally, because HYSYS can calculate the
chemical activity of each component in the mixture based on the fugacity of the
mixture or pure component.
Case example
A feedstock of pure n-butane (100
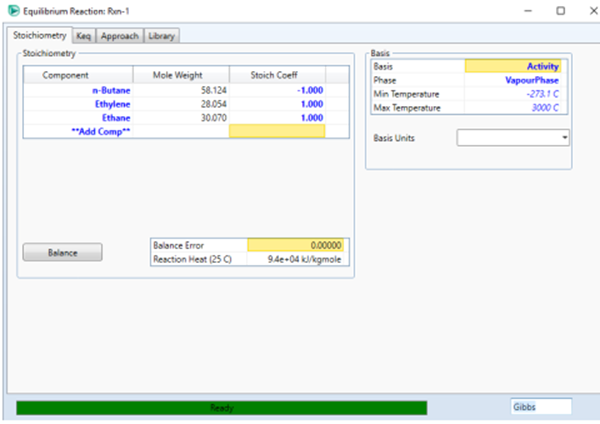
kmol/hr) is cracked at 750 K and 1.2 bar to produce olefins. Only two reactions
have favorable equilibrium conversions at these conditions:
If these reactions reach
equilibrium, what is the product composition? Use Peng-Robinson Fluid Package
The steps in completing this simulation are
the same as working on the conversion reactor, only different in the selection
of the reaction type!
Add reactions in the same way as
the conversion reactor, but this time select Equilibrium reaction.
Define the stoichiometry of each
component as below. Also, define reaction 2 in the same way. When finished
click Add to FP
and continue with the simulation process.
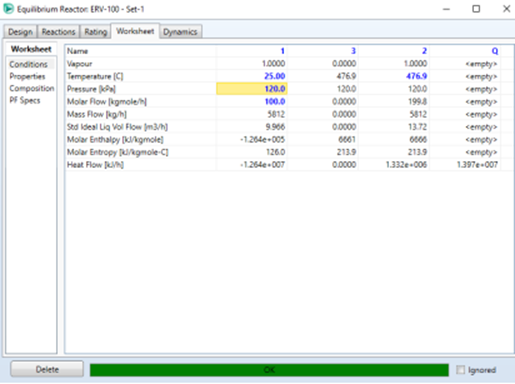
Define the operating conditions
used on the Worksheet tab as shown below
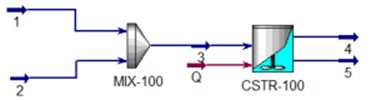
3. Ideal CSTR Reactor (Continuous Stirred-Tank Reactor)
CSTR reactors are one of the most
commonly used reactor types in chemical process simulations. In this reactor,
chemical reactions occur in a tank with continuous mixing. The reactants are
added to the tank and mixed continuously. The advantage of this reactor is that
the temperature and concentration can be kept stable. However, this reactor is
more suitable for reactions with slow reaction kinetics.
The CSTR reactor assumes perfect
mixing in each part of the reactor volume and the outflow conditions are the
same as the conditions inside the reactor. Reactions that can be used for
modeling this type of reactor are kinetic reactions and heterogeneous catalysis
In the CSTR reactor, the conversion of the reactor is also affected by the
reactor volume, so volume geometry information must be given. Depending on the
reactor geometry, at least 2 pieces of information must be provided, either
volume, height, or diameter.
Required input data
Propylene Glycol can be produced
from the reaction between Propylene Oxide and water as below:
With the following reaction
kinetics data
with the base unit in lb mol/ft3
and the rate unit in lb mol/ ft3 hr. Propylene Oxide (150 kmol/hr) and water
(200 kmol/hr) at 25 C and 101.3 kPa. enter the reactor through 2 different
streams which will then be mixed using a mixer. The CSTR reactor operates at a
pressure of 101.3 kPa and 25°C with a volume of 5 m3. What is the rate of
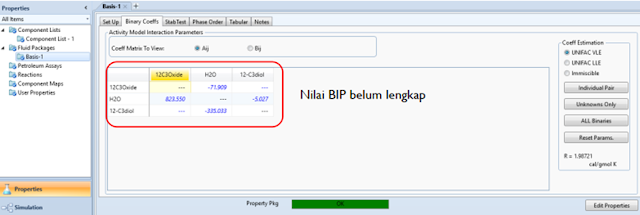
Propylene Glycol produced? Select UNIQUAC as the fluid package.
Estimation of Binary Interaction
Parameters that are not yet available.
Estimation of unavailable Binary
Interaction Parameters
- Unavailable BIP values can be
estimated with 3 method options
- UNIFAC VLE, LLE or immicisble
solution.
- In this case, we choose UNIFAC
VLE
- Click on unknowns only
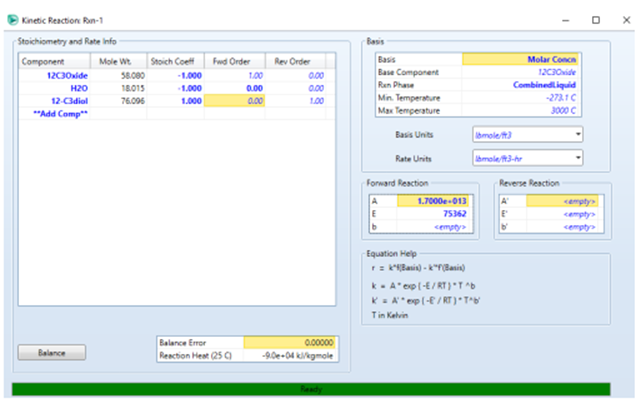
Provide reaction input by first
selecting the reaction type "Kinetics", and input as follows
The forward order value for H2O
is 0 because H2O is given in excess so the reaction rate
only depends on the concentration of propylene oxide.
Make the PFD as shown below. The
way to input the reaction in the reactor operation is the same as in the
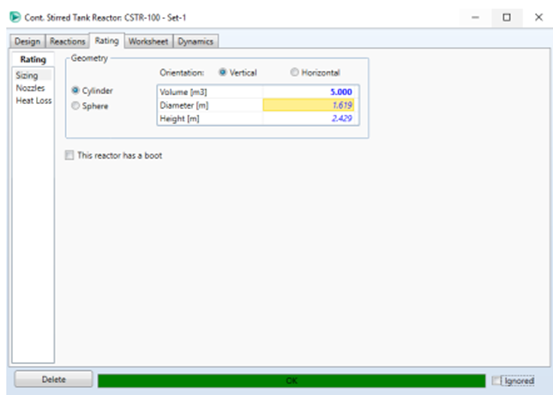
conversion reactor. To enter the reactor volume, click on Rating → Sizing.
Since the reactor operates in an
isothermal state (25 OC), on the worksheet enter 25 oC for stream temperature 5 or 4.
Reaction conversion can be seen
on the Reaction → Result
tab.
After converging try to increase
the reaction temperature to 75 oC, then check the conversion
of the reaction.
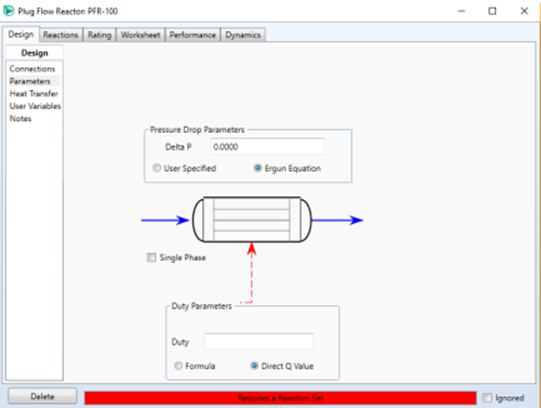
4. Ideal PFR (Plug Flow Reactor) Reactor
In addition to the CSTR reactor,
another type of reactor commonly used in industry is the PFR. This reactor is
composed of a cylindrical pipe and normally operates in a steady state like the
CSTR reactor. PFR reactors are generally used for reactions in the gas phase.
The PFR reactor describes the
chemical reaction process in pipe flow without mixing. This reactor assumes
that reactions occur in sequential order as materials pass through the reactor.
This reactor is suitable for reactions with fast reaction kinetics and
sequential reactions. In the PFR simulation with HYSYS, the reaction types that
can be selected are kinetic and heterogeneous catalysis. Just like in CSTR, the
conversion value of the PFR reactor is also affected by the reactor volume, so
information about the reactor geometry must be provided.
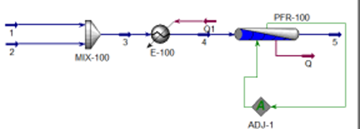
For the reaction below, the
selected reactor is PFR. The operating conditions of the reactor are set at
400°C, and the pressure drop of the reactor is calculated by Ergun's equation.
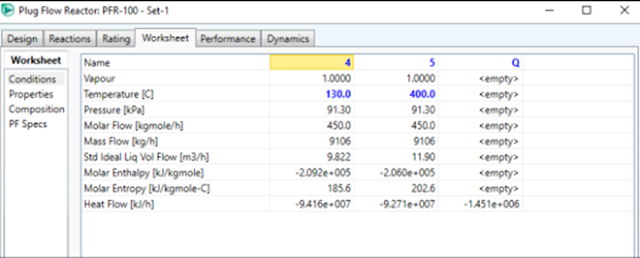
Before entering the reactor, the mixed feed stream is preheated to 130°C.
Initial feed conditions for CO were at 25°C, 101.3 kPa pressure, and 100 kmol/h
flow rate. While H2O is at a temperature of 120°C, pressure of 101.3 kPa, and
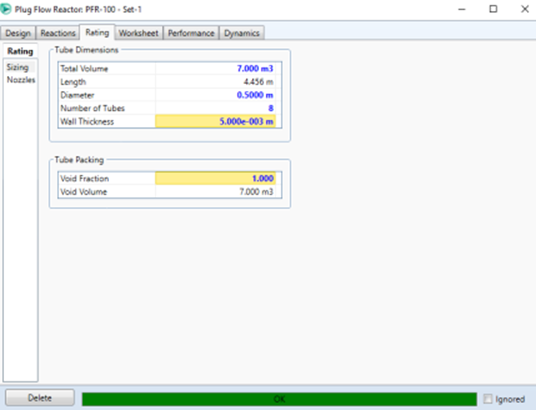
flow rate of 350 kmol/hour. The reactor volume used is 7 m3 with a diameter of
5 m and the number of tubes is 8. Choose NRTL as a fluid package and
Peng-Robinson as a vapor model.
The reaction kinetics data are
presented as follows:
Base: mole fraction of CO, with
rate unit kg mol/m3 s.
Forward reaction: A = 5.9 x
108, E = 1.2 x 105 kJ/kmol
Estimation of unavailable Binary
Interaction Parameters
Provide reaction input by first
selecting the reaction type Kinetics, and input as follows.
- Base: Mole Fraction
- Reaction Phase on Vapor phase
Pressure drop in the reactor can be input directly or can be estimated using the Ergun equation
Reactor geometry can be input as
follows Volume: 7 m3; Diameter 0.5 m; Number of Tubes: 8
Set the reactor exit temperature
at 400 oC
by giving a Stream
temperature input of 5.
When designing chemical processes
or trying to understand reaction kinetics, an understanding of the appropriate
reactor type is important. Aspen HYSYS provides a powerful tool for exploring
these different reactor types, and this is only a small selection of the
options available. The selection of the right reactor depends largely on the
type of reaction, kinetics, and operating conditions you are dealing with.